Rheumatoid arthritis is a chronic condition that causes the body’s immune system to attack the joints, resulting in inflammation, swelling and pain.
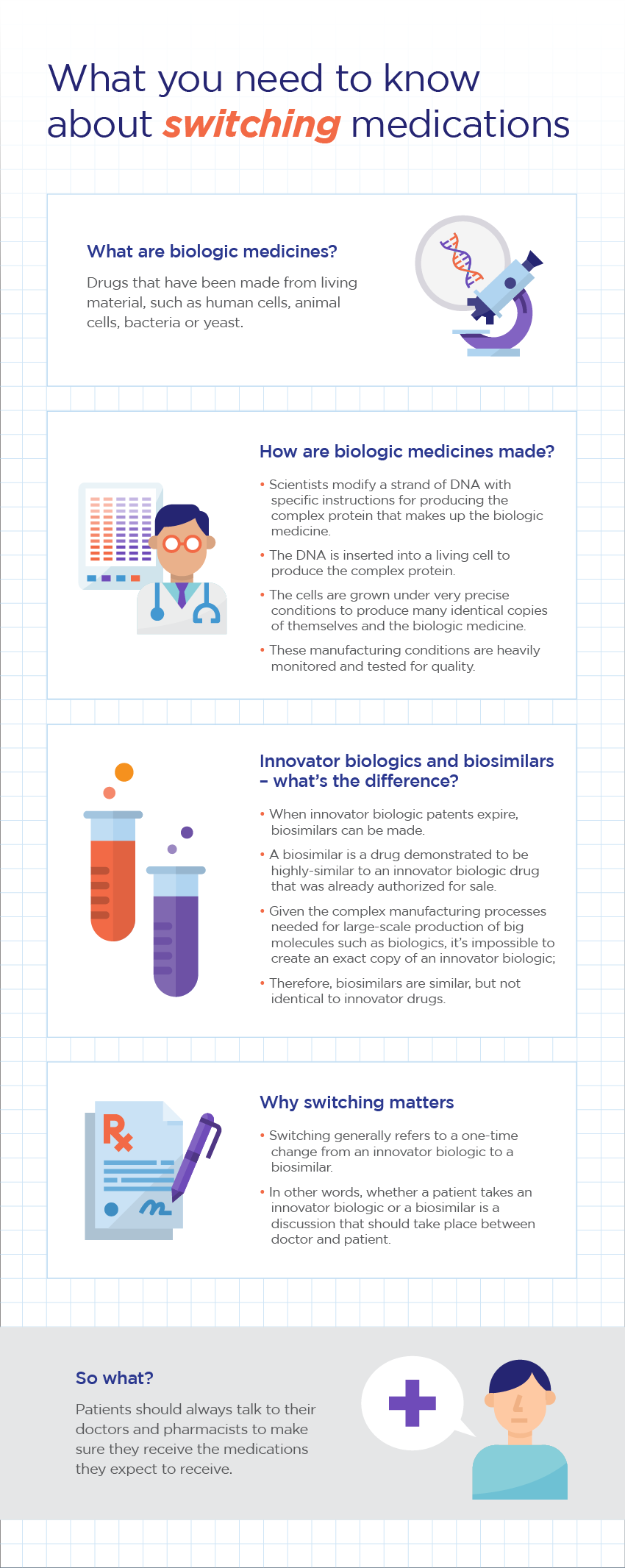
Finding an effective medication that helps manage the condition can, for many patients, be a lengthy process. Some patients are prescribed biologic medications, of which there are two different kinds: innovator biologics and biosimilars. The nature of their differences means that physicians and patients need to carefully consider a switch from one to the other.
As the name suggests, biosimilars are similar but not identical to the innovator biologics they intend to copy. Both types of drugs are produced from living cells using very precise manufacturing processes. Given their inherent complexity and the size of the molecules, it is impossible for a biosimilar to be an exact replica of the innovator biologic.
Some patients who are on one product may be faced with a decision to switch medications, due to costs, changes in reimbursement, or other non-medical issues. Patients should be fully informed before making any decisions related to their medications.
The infographic below helps patients and caregivers understand the differences between innovator biologics and biosimilars.
- Marrow T. Defining the difference: what makes biologics unique. Biotechnology Healthcare. September 2004: 24-29;
- https://safebiologics.org/wp-content/uploads/2017/11/ROADSHOW-1P-EXTRAPOLATION-091417.pdf
- https://www.canada.ca/en/health-canada/services/drugs-health-products/biologics-radiopharmaceuticals-genetic-therapies/applications-submissions/guidance-documents/fact-sheet-biosimilars.html
- https://arthritis.ca/understand-arthritis/types-of-arthritis/rheumatoid-arthritis
- https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhp-mps/alt_formats/pdf/brgtherap/applic-demande/guides/biosimilars-biosimilaires-qa-qr-eng.pdf


